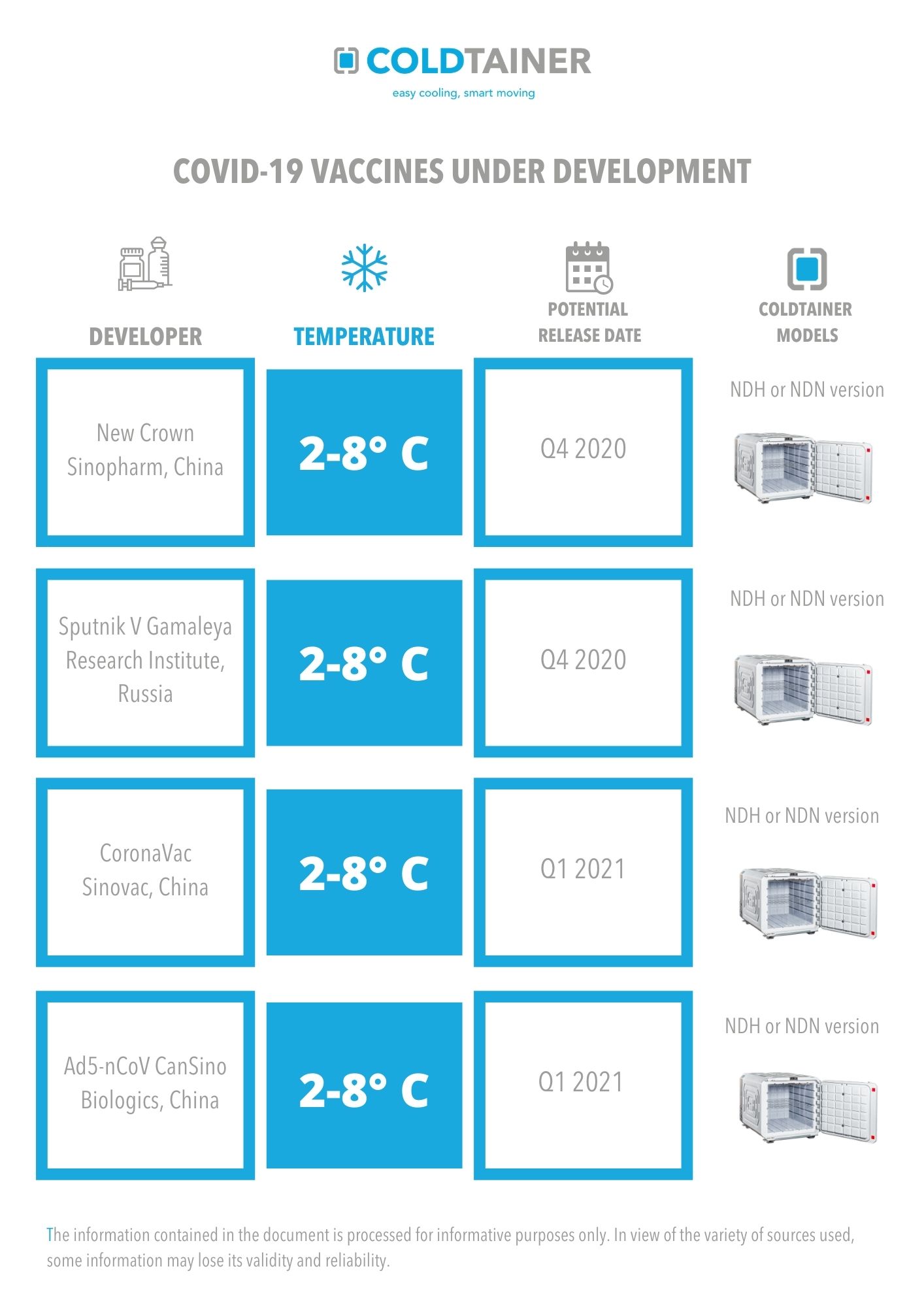
As New Vaccines are Developed, Coldtainer Stands Ready

With the welcome news that multiple vaccines are in development for the Covid-19 pandemic, Coldtainer stands ready to assist any and all efforts to get these medicines to the people that need them. The vaccines’ distribution will be the next great challenge for the logistics world. The entire transport sector will be involved and Coldtainer’s GDP-compliant refrigerated isothermal containers are perfect for the transport and storage of vaccines and pharmaceutical supplies.
Coldtainer mobile refrigerators are safe and effective to transport medicines, vaccines, biological samples to be analyzed, blood and derivatives. Depending on the models, the NDH and FDH versions are available, with an automatic cooling/heating system to guarantee the products transport at a constant temperature, regardless of the external environmental conditions, in compliance with the regulations in force (GDP 2013/C 68/01, WHO 961/2011). Coldtainer mobile refrigerators, being suitable for the transport of any type of product, are not classifiable as “Medical Devices” and are not subject to specific norms or approvals.
Based on the information currently available, we have provided an overview of the main vaccine candidates, as well as the specific Coldtainer model best used for transport.